BillionToOne is a precision diagnostics startup that has rapidly risen to prominence with its groundbreaking UNITY prenatal testing platform. Founded in 2016 and headquartered in Silicon Valley, the company’s mission is to make molecular diagnostics more accurate, efficient, and accessible.
BillionToOne’s flagship UNITY Screen has redefined prenatal care by combining traditional non-invasive prenatal testing (NIPT) for chromosomal abnormalities with screening for single-gene recessive disorders – all from a single tube of the mother’s blood. This unique approach addresses critical gaps in prenatal screening and exemplifies the company’s innovative spirit.
Over the past decade, BillionToOne has evolved from a scrappy Y Combinator-backed idea into a high-growth industry leader serving both the prenatal and oncology diagnostics markets.
By 2025, the company has processed over 1,000,000 patient tests and achieved $209 million in last-twelve-months revenue, reflecting an 84% year-over-year growth and emerging profitability.
In this report, we present a comprehensive startup story and business analysis of BillionToOne, covering its origin, founders, business model, financial trajectory, competitive landscape, and the products that underpin its success.
Founding Story of BillionToOne
BillionToOne’s story begins in 2016 with three co-founders determined to solve a vexing challenge in prenatal genetics. Working out of half a bench in a Stanford University incubator, the founders – Oguzhan Atay, David Tsao, and Sukrit Silas – set out to develop a method to detect the tiniest genetic signals in a blood sample.
Early fundraising was tough: securing the first $300,000 in seed funding proved harder than any subsequent millions. In fact, the very name “BillionToOne” encapsulates their technological breakthrough and frugality. It refers to their platform’s ability to detect a single DNA letter among the three billion letters in the human genome, and also nods to the fact that “billiontoone.com” was an available domain they scooped up for just $12 rather than paying for a pricier name. This frugal, resourceful mindset defined BillionToOne’s early growth.
From the outset, the team demonstrated relentless resourcefulness. In the company’s infancy, the founders personally traveled to Turkey and India to collect clinical samples for their studies, targeting regions where beta-thalassemia and other genetic diseases are common. Smuggling reagents through customs and hand-carrying blood samples was nerve-wracking, but each sample was vital data for refining their technology.
By 2017, BillionToOne was accepted into Y Combinator’s Summer ’17 batch, giving it a boost of credibility and initial capital as a Silicon Valley startup. A pivotal moment came in 2018, when the company received an NIH grant and partnered with Baylor College of Medicine to conduct the first clinical study of a single-gene non-invasive prenatal test. These early trials validated BillionToOne’s patented molecular counting method and showed it could accurately detect fetal single-gene disorders from maternal blood – a feat previously thought impossible.
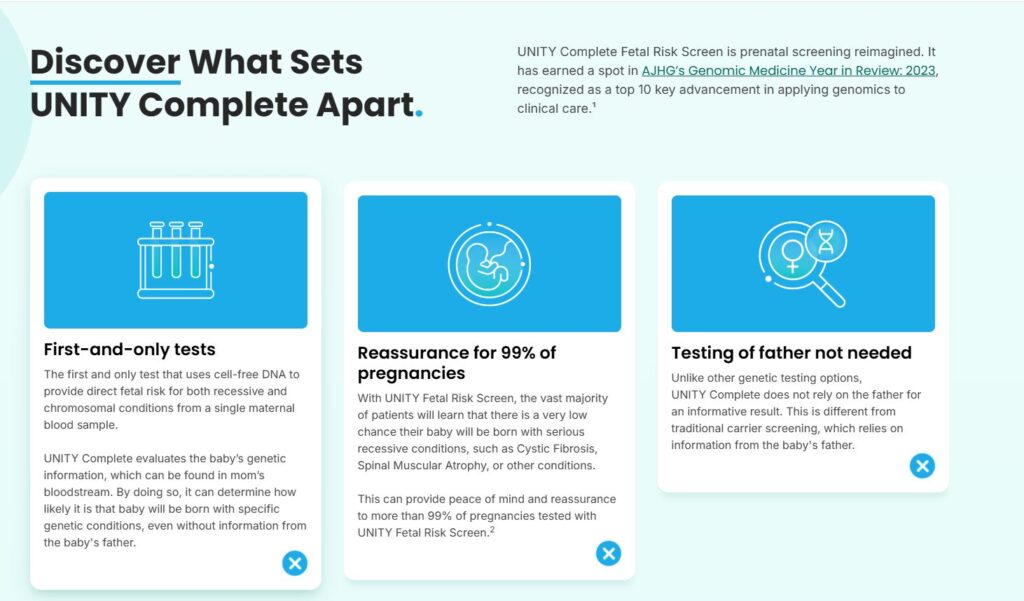
In 2019, BillionToOne officially launched UNITY, its first product, aiming to revolutionize prenatal screening. UNITY introduced a two-step workflow: first, test the mother’s blood for carrier status of recessive diseases; if she’s a carrier, then analyze the cell-free fetal DNA in the same sample to determine if the baby is at risk. This clever approach eliminated the need for paternal testing in most cases and circumvented invasive procedures like amniocentesis.
By mid-2019 the company raised a $15 million Series A round to fuel UNITY’s launch. As demand quickly grew, BillionToOne expanded its CLIA-certified laboratory and by summer 2020 was delivering UNITY tests nationwide. Even the COVID-19 pandemic did not stall its momentum – the team set audacious goals and achieved them. When clinics limited rep visits in 2020, BillionToOne procured scarce N95 masks so their sales team could safely continue reaching OB/GYN offices.
The result: test volumes still grew more than 10-fold in 2020, despite the pandemic’s challenges. This can-do ethos of focusing on mission-critical goals and ignoring distractions (like premature M&A talks or “me-too” products) allowed BillionToOne to accelerate through early obstacles.
Founders of BillionToOne
Oguzhan Atay, Ph.D. (Co-founder & CEO)

Born and raised in Turkey, Oguzhan Atay is the visionary leading BillionToOne. He earned his undergraduate degree in Molecular Biology from Princeton University (graduating valedictorian of his class) and a Ph.D. in Systems Biology from Stanford. With a multidisciplinary background spanning molecular biology, computer science, physics, and applied math, Atay envisioned applying quantitative principles to diagnostics.
At Stanford, he met his future co-founders and cultivated the idea of a “molecular counter” that could identify disease-causing DNA fragments amid a sea of normal DNA. As CEO, Atay steered BillionToOne from inception through rapid growth. He is known for setting bold targets – for example, in 2020 he challenged the team to process 1 million tests by 2025, a goal achieved ahead of schedule.
Atay’s relentless focus on execution and his “pressure is a privilege” philosophy shaped the company’s high-performance culture. Under his leadership, BillionToOne also navigated strategic expansions, including the foray into oncology diagnostics and preparations for an IPO.
David Tsao, Ph.D. (Co-founder & CTO)

David Tsao is the technological brain behind BillionToOne’s platform. A physicist-turned-biotech innovator, Tsao earned a B.A. in Physics from Princeton (with honors) and a Ph.D. in Bioengineering from Rice University. His eclectic career before BillionToOne included experiences as a spacecraft engineer and software developer.
Tsao is the original inventor of BillionToOne’s proprietary Quantitative Counting Templates (QCT) molecular counting method. He led the development of the core DNA counting algorithms and bioinformatics pipeline that enable single-gene mutation detection in cell-free DNA. As CTO, Tsao authored the foundational patents and spearheaded the clinical validation studies that proved the accuracy of UNITY.
He continues to oversee R&D, translating the company’s scientific vision into robust assays. Colleagues describe Tsao as a polymath who brings engineering discipline to biology – an approach that helped build BillionToOne’s technology platform from the ground up. His diverse skillset (ranging from physics to genomics) has been instrumental in solving complex technical challenges and differentiating the company’s products.
Sukrit Silas, Ph.D. (Co-founder & Scientific Advisor)

Sukrit Silas co-founded BillionToOne during his doctoral research at Stanford. Working in the lab of Nobel laureate Andrew Fire, Silas made a breakthrough discovery in how bacteria use CRISPR systems to fight RNA viruses. This scientific curiosity and inventive mindset carried into BillionToOne’s inception.
Silas was featured in Forbes 30 Under 30 (2019) for his work with BillionToOne, which by then had raised an initial $2.5 million to develop diagnostics for beta-thalassemia and Down syndrome. With a Ph.D. in Chemical & Systems Biology from Stanford, Silas brought deep expertise in genetics and assay design to the founding team.
He helped design early experiments and contributed to the single-gene NIPT concept. After the company’s early stage, Silas transitioned to focus on academic research (launching the Silas Lab at Gladstone Institutes) but remains an advisor and equity holder in BillionToOne. His scientific acumen and discoveries laid some of the groundwork for the company’s innovations.
Together, the founding trio’s complementary strengths – Atay’s bold leadership, Tsao’s engineering prowess, and Silas’s scientific creativity – formed the backbone of BillionToOne’s brand and culture. They instilled core principles still evident today, such as a “patients first” mentality (the founders personally reviewed early patient reports to ensure clarity and compassion) and a hire-the-best philosophy (accepting only the top 1% of job applicants to build an elite team). These values have guided BillionToOne from a tiny lab bench operation into a company of over 500 employees as of late 2024, poised to reshape the future of molecular diagnostics.
Business Model of BillionToOne
BillionToOne operates as a molecular diagnostics service provider, offering advanced genetic tests through a centralized laboratory model. Its business model revolves around developing unique tests and performing them in-house on patient samples sent by healthcare providers. For its flagship UNITY prenatal test, the company’s primary customers are OB/GYN clinics, maternal-fetal medicine specialists, and clinical laboratories that order the test for expectant parents. BillionToOne supplies blood collection kits to these providers, who draw a tube of the pregnant mother’s blood and ship it to BillionToOne’s CLIA-certified lab in California. There, BillionToOne’s scientists extract cell-free DNA and apply the proprietary QCT sequencing assay to detect fetal risks. Test results and interpretations are delivered back electronically to physicians (and often accompanied by genetic counseling services for patients as needed).
Revenue is generated on a per-test fee basis, primarily via reimbursements from insurance payors and healthcare systems. BillionToOne has strategically worked to secure insurance coverage for its tests – by the end of 2024, the UNITY prenatal screen was covered for over 225 million insured lives in the U.S., thanks to contracts with major private insurers and Medicare/Medicaid policies. This broad payor coverage enhances access for patients and provides more predictable revenue streams. The company’s billing model typically involves insurance claims (where available) or direct billing to clinics/patients when necessary, similar to other diagnostics providers. Importantly, UNITY falls under recommended prenatal care guidelines (ACOG recommends carrier screening for all pregnant women and NIPT for all pregnancies), which has helped drive both demand and insurance adoption.
A key element of BillionToOne’s model is maintaining control over the testing process to ensure quality and efficiency. Unlike some competitors who license their technology to third-party labs, BillionToOne runs tests in its own expanding lab facilities. In 2021, the company built out a new 36,000 sq ft laboratory to scale up capacity. It also employs a growing commercial team of field representatives who educate providers and facilitate test adoption nationwide. By 2020, BillionToOne had over 50 sales reps and plans to continue growing the salesforce to reach more clinics. This direct sales approach allows the company to capture clinician mindshare and differentiate UNITY’s benefits.
Notably, BillionToOne demonstrated agility in its business model during the COVID-19 pandemic. In 2020, when traditional prenatal testing volumes were threatened by lockdowns, the company rapidly developed a novel COVID-19 diagnostic assay (qSanger-COVID-19) that used its DNA counting technology to enable high-throughput testing. It obtained FDA Emergency Use Authorization and, through a partnership with Swift Biosciences, scaled manufacturing of this test. This pivot not only contributed testing capacity during a crisis but also generated additional revenue at a critical time. More fundamentally, it showcased the versatility of BillionToOne’s platform (able to pivot from prenatal genetics to infectious disease) and the company’s ability to execute under pressure. Ultimately, however, prenatal screening remained the core focus – as clinics reopened, BillionToOne doubled down on UNITY and its long-term roadmap.
Operationally, the company emphasizes cost discipline and scalability. Management has taken a stepwise approach to automation, incrementally bottlenecking and improving lab processes rather than overspending on fully automated setups from the start. This lean operations philosophy yielded a competitive cost of goods; in one year, the team drove a ~20% reduction in per-test COGS by systematically optimizing workflows. Such efficiencies, combined with increasing volume, have allowed BillionToOne to improve gross margins (53% in 2024) and approach profitability. By mid-2025 the company achieved a positive non-GAAP operating income for the first time – a rarity among high-growth diagnostics startups.
In summary, BillionToOne’s business model is built on high-impact proprietary tests, an in-house testing service with strong quality control, insurance-friendly economics, and a focus on executing at scale while containing costs.
Revenue Streams of BillionToOne
BillionToOne’s revenues are primarily driven by its clinical testing services, with the UNITY prenatal testing platform constituting the lion’s share. As of 2025, the company has two main commercial segments:
-
Prenatal Screening Services: This is the dominant revenue stream. It encompasses all UNITY prenatal test panels and related services. Physicians order UNITY Complete (which covers aneuploidies and single-gene conditions) or specific UNITY sub-tests (e.g. just aneuploidy + carrier screen, or the Rh incompatibility test), and BillionToOne bills per test. The price point per test is typically reimbursed by insurance; for example, Medicaid and private insurers cover UNITY’s various components according to medical necessity guidelines (Down syndrome screening, carrier screening, etc.). Uptake has grown explosively – the company processed over 500,000 prenatal tests in 2023 alone. Correspondingly, prenatal screening revenue reached ~$153 million in 2024, more than doubling from $72M in 2023. This growth reflects both higher test volumes and improving reimbursement rates as coverage expanded. The prenatal segment includes not just the laboratory testing, but also ancillary services that add value: physicians receive comprehensive reports, and patients/clinicians have access to BillionToOne’s genetic counseling team for consultation on results. These services enhance the value proposition of UNITY and help drive adoption (though they are typically bundled rather than separate revenue lines).
-
Oncology and Research Testing: A newer but rapidly emerging revenue source is BillionToOne’s oncology diagnostics, branded under the Northstar platform. In 2023, the company began offering Northstar Select (tumor genomic profiling) and Northstar Response (treatment response monitoring) assays to oncology centers and pharmaceutical research partners. Initially launched for research use with leading cancer institutes, these tests started generating modest revenue through research collaborations and clinical trial services. For instance, BillionToOne partnered with UC San Diego and others to validate Northstar assays on patient samples. By late 2024, the company was commercializing these tests clinically, aiming at oncology clinics treating late-stage cancer patients. While oncology represented a small portion of 2024 revenue (~$1–2M), it is expected to be a significant growth driver going forward. In addition, BillionToOne has earned some revenue from clinical trial services – providing testing for pharmaceutical trials (e.g., using Northstar Response to monitor patients in drug studies). These partnerships not only bring in service revenue but also position the company for companion diagnostic opportunities in the future.
Historically, BillionToOne had other one-time or minor revenue sources. Its COVID-19 testing in 2020–2021 contributed a short-term boost (the company at one point touted the capacity to run up to one million COVID tests per day with its extraction-free protocol). However, by 2022 this was phased out as demand receded and focus returned to core products. The company does not currently generate revenue from licensing its technology; instead, it has opted to keep its IP in-house and monetize through performing tests. The strategy has paid off – total revenues climbed to $152.6M in 2024 (113% annual growth), and on a last-twelve-months basis topped $209M by mid-2025.
Table 1: Funding History of BillionToOne (2016–2024)
| Date | Round | Amount Raised | Key Investors | Notable Use/Purpose |
|---|---|---|---|---|
| 2017–2018 | Seed / Angel | ~$2.5 Million | Y Combinator (S17 batch), 500 Startups, etc. | R&D of molecular counting, early hires. |
| Mar 2019 | Series A | $15 Million | Hummingbird Ventures, NeoTribe Ventures (co-leads); Y Combinator, 500 Startups Istanbul, Libertus, Civilization Ventures. | Launch of UNITY prenatal test in U.S. |
| Mar 2020 | Series A+ | $15 Million | Hummingbird & NeoTribe (follow-on); Pacific 8, HOF Capital, others. | Expand UNITY to all U.S. states; scale CLIA lab 20× capacity. |
| Jul 2021 | Series B | $55 Million | Hummingbird (lead); Four Rivers, Norwest, Neotribe, Libertus, Y Combinator. | Build 36k sq ft lab; grow commercial team nationally. |
| Mar 2022 | Series C | $125 Million | Adams Street Partners & Hummingbird (co-leads); Baillie Gifford, Neotribe, Norwest, Fifty Years, Pacific 8, Time BioVentures, Libertus, Civilization Ventures. | Develop oncology (Northstar) tests; expand sales/clinical teams. |
| Jun 2024 | Series D | $130 Million | Premji Invest (lead); Neuberger Berman (major new); also Adams Street, Baillie Gifford, Hummingbird, Libertus, Fifty Years, etc. | Scale prenatal and oncology businesses; reached >$1B “unicorn” valuation. |
| Sep 2024 | Venture Debt | $140 Million | (Lenders undisclosed; structured debt financing) | Strengthen balance sheet pre-IPO; support lab expansion and ops |
Sources: Company press releases and news reports.
BillionToOne’s funding trajectory highlights investor confidence in its vision. After a modest seed round, the company attracted larger financings as it hit technical and commercial milestones. Notably, by mid-2024 BillionToOne achieved unicorn status, with its Series D valuing it above $1 billion. Cumulatively, the company has raised roughly $400 million in capital (equity and debt), ensuring ample resources to execute its growth plans.
The proceeds have been used to expand laboratory infrastructure, accelerate R&D (e.g. clinical studies for new tests), and build out the operational capacity to handle rising test volumes. By late 2025, BillionToOne signaled readiness for the public markets, confidentially filing for an IPO on Nasdaq (ticker “BLLN”). This move positions the company to access even greater capital for its next chapter, while its founders retain controlling interest via Class B shares.
Competitors of BillionToOne
BillionToOne operates across prenatal genetics and precision oncology, so its competitors come from both large diagnostic companies and newer genomic innovators. Below is a concise overview of key competitors in NIPT and oncology, and how BillionToOne differentiates.
Prenatal Screening Competitors (NIPT)
1) Natera (Panorama)

Natera is a market leader in NIPT with its SNP-based test, Panorama, covering common aneuploidies and optional microdeletions. While widely adopted, Panorama does not screen single-gene disorders in the fetus without paternal testing. BillionToOne’s UNITY uniquely screens recessive genetic diseases directly from maternal blood (e.g., CF, SMA, sickle cell, thalassemias) without a second parent test. Natera typically requires multiple separate tests, whereas UNITY delivers a combined NIPT + carrier screen in a single report. Natera has scale, but UNITY’s integrated approach has driven fast market share growth.
2) LabCorp (MaterniT21)

LabCorp provides MaterniT21, one of the earliest NIPTs, screening for trisomies 21/18/13 and some microdeletions. As a national lab, it benefits from broad distribution, but its offering is focused on chromosomal aneuploidies only. UNITY competes by offering expanded features in one test: aneuploidies, single-gene disorders, and optional Rh status, eliminating multiple workflows. LabCorp’s scale keeps costs low, but BillionToOne’s focused innovation allows faster development of specialized tests.
3) Myriad Genetics (Prequel)

Myriad’s Prequel test features very low test failure rates and early testing (from week 8), covering standard NIPT panels with microdeletions. Single-gene screening requires pairing with Myriad’s Foresight carrier test and follow-up paternal testing if needed. UNITY bypasses this step by direct fetal mutation assessment when the mother is a carrier, reducing time and complexity. UNITY’s menu also includes Fragile X, 22q11.2 deletion, and additional rare disorders. Myriad is large and established, but UNITY’s efficiency and published clinical data have accelerated adoption.
4) Quest Diagnostics (QNatal Advanced)

Quest offers QNatal, a national NIPT for standard aneuploidies. It competes on accessibility through routine OB workflows, but has limited capabilities beyond chromosomal testing and often depends on partner labs for advanced genetics. UNITY differentiates by offering a truly integrated platform, rather than standalone NIPT.
Other players include Roche’s Harmony (more active in Europe/Asia) and Juno Diagnostics, which is exploring at-home NIPT. However, as of 2025 no competitor offers a single integrated NIPT covering aneuploidies + recessive diseases + fetal antigen status on a single blood draw—an area where BillionToOne remains differentiated.
Oncology Diagnostics Competitors (Liquid Biopsy)
1) Guardant Health (Guardant360, Reveal)

Guardant is a leader in comprehensive genomic profiling (CGP) and minimal residual disease (MRD). Guardant360 profiles 70+ genes to inform treatment decisions; Reveal focuses on MRD in early-stage cancers. BillionToOne’s Northstar Select competes directly in CGP with an emphasis on higher sensitivity at low variant frequencies via QCT technology. Northstar Response uses a methylation-based monitoring approach, offering a quantitative view of tumor burden. Guardant remains the dominant player, but Northstar aims to win on ultra-sensitivity and precise monitoring.
2) Foundation Medicine (FoundationOne Liquid CDx)

Foundation offers an FDA-approved liquid biopsy for advanced cancers, with strong clinical adoption. Northstar competes by offering two complementary assays—Select (mutation profiling) and Response (disease monitoring)—giving physicians both actionable insights and therapy tracking. If Northstar proves earlier response detection, it could offer an edge over pure profiling tests.
3) Natera (Signatera)

Natera also competes in oncology through Signatera, a personalized MRD test used after surgery to detect recurrence. Signatera targets early recurrence, while Northstar focuses on real-time monitoring during therapy in metastatic settings, so there is partial overlap. Both leverage cell-free DNA expertise, but BillionToOne’s methylation-driven, tissue-agnostic method is strategically distinct.
4) Grail (Galleri)

Grail’s Galleri screens asymptomatic individuals for early cancer detection using multi-cancer signals. While not a direct competitor to Northstar (which targets diagnosed patients), Grail represents a major force in liquid biopsy innovation. BillionToOne positions Northstar in a different clinical pathway: precision treatment guidance rather than population-level screening.
Competitive Advantage
BillionToOne’s edge comes from the combination of breakthrough technology, differentiated products, strong execution, and a focused strategy. Below are the core drivers of its market advantage.
1) Proprietary Molecular Counting Technology
BillionToOne’s platform is built on its QCT™ (Quantitative Counting Template) technology, a molecular counting method capable of single-digit DNA quantification. QCT removes PCR biases and can detect one mutated molecule among millions, increasing cell-free DNA resolution by 1000x. This enables single-gene detection in prenatal testing and ultra-sensitive mutation and methylation detection in liquid biopsy. While competitors focus on larger chromosomal changes, QCT makes single-mutation NIPT and precise therapy monitoring possible. The technology is backed by published validation, creating both a performance advantage and a defensible IP moat.
2) First-Mover in Single-Gene NIPT
UNITY is the first prenatal screen to measure fetal risk for recessive disorders (CF, SMA, sickle cell, thalassemias, etc.) without paternal DNA. This solves a critical workflow gap—paternal samples are often unavailable, reducing detection rates. UNITY boosts detection from ~41% under the two-parent model to >98% with a single maternal blood draw, significantly improving clinical utility. This simplified logistics + superior sensitivity has accelerated provider adoption. As an early mover, BillionToOne now has large datasets and real-world clinical experience that new entrants will take years to match.
3) Comprehensive Test Offerings
BillionToOne consolidated multiple workflows into one unified test. UNITY Complete reports on aneuploidies, sex chromosome conditions, 22q deletion, maternal carrier status for multiple single-gene disorders, and fetal antigen/Rh status in a single result. This reduces fragmented ordering and often lowers total cost versus running separate panels. In oncology, Northstar Select + Northstar Response allows oncologists to profile mutations and monitor tumor burden through one platform, instead of using multiple vendors. This integrated offering is a clear differentiator for providers seeking clean workflows and fewer touchpoints.
4) Clinical Validation and Publication
BillionToOne has invested heavily in clinical evidence, publishing studies validating QCT, UNITY accuracy, and the economic value of single-gene NIPT. Its data has influenced national guidelines (e.g., ACOG/ACMG updates supporting fetal antigen NIPT), which is rare for a young diagnostics company. These endorsements build trust with clinicians, support reimbursement discussions, and raise barriers to entry—competitors must generate comparable evidence to claim similar performance. Scientific credibility strengthens both adoption and defensibility.
5) Rapid Growth with Financial Discipline
The company pairs hyper-growth with strong financial execution. From 2021–2024, revenue grew triple-digit CAGR, reaching $150M+, while losses narrowed sharply, with the business approaching breakeven by mid-2025. Unlike competitors that pursue scale at any cost, BillionToOne improved gross margins, operating efficiency, and focused only on core priorities. The result is 80%+ YoY growth with improving profitability, giving the company resilience in a capital-intensive industry where many peers burn cash. This execution track record is attractive to both providers and investors.
6) Mission-Driven Culture and Talent
BillionToOne’s culture emphasizes ownership, speed, and top-tier talent, hiring selectively across molecular biology, engineering, and clinical genetics. This allows rapid product iteration, high-quality clinical support, and close scientific oversight of complex cases. The team’s agility has enabled achievements like deploying a COVID testing program in weeks. Its mission—“removing the fear of the unknown”—drives strong internal alignment and a customer-centric mindset, making the experience attractive for clinicians and patients. Culture and talent become a soft moat that is difficult to replicate.
Overall, BillionToOne’s advantage lies in breaking technical barriers (single-molecule counting), delivering fully integrated tests, and executing with discipline and focus. These strengths have allowed it to challenge much larger incumbents and rapidly gain market share. As it moves deeper into oncology, the same strengths—platform technology, integrated product design, clinical evidence, and operational excellence—position it to compete effectively against entrenched diagnostic leaders.
Products and Services
BillionToOne’s portfolio reflects its strategy of using molecular counting to build precision diagnostics in high-impact clinical areas. Its offerings fall into two categories: Prenatal (UNITY) and Oncology (Northstar).
1) UNITY Prenatal Testing Platform
UNITY is BillionToOne’s flagship product line, built around integrated prenatal screening powered by QCT technology. The platform is sold as multiple panels, with UNITY Complete® representing the full offering.
2) Combined NIPT + Carrier Screening
UNITY is the only test that combined standard NIPT with maternal carrier screening and reflex fetal testing in a single blood draw. It screens for aneuploidies (e.g., Down syndrome) while simultaneously testing for recessive single-gene conditions like CF, SMA, sickle cell disease, and thalassemias, with optional Fragile X. If the mother is a carrier, UNITY directly analyzes fetal DNA for that mutation from the same sample—removing the need for paternal testing or invasive procedures. This model replaces a multi-step workflow with one streamlined test, providing two layers of information from one draw.
3) Aneuploidy Screening
UNITY covers trisomies 21, 18, and 13 with accuracy comparable to leading NIPTs. Providers can add sex chromosome analysis (e.g., Turner/Klinefelter) and 22q11.2 deletion screening to tailor depth based on risk. The turnaround time is typically 1–2 weeks, consistent with other top-tier NIPT providers.
4) Fetal Antigen (Rh & Other) Testing
UNITY also offers fetal blood antigen testing, a key differentiator. It identifies fetal RhD status from maternal blood with >99.9% accuracy, informing the use of RhoGAM in Rh-negative pregnancies. This enables targeted administration rather than routine prophylaxis, especially useful during RhoGAM supply shortages. UNITY also supports other antigen screens (e.g., C, c, E, Kell, Duffy) for alloimmunized patients. By 2023, UNITY had supported 80,000+ cases for fetal antigen management, a capability few competitors offer.
5) Clinical Reporting & Services
Report outputs present clear risk assessments and guidance on next steps. If a result is high-risk, genetic counselors support clinicians and patients—often within one business day—integrating interpretation and counseling into the testing experience. UNITY is positioned as a full-service solution, not just a lab result.
UNITY has expanded to multiple orderable panels (e.g., UNITY Complete, single-gene + aneuploidy, etc.), reflecting continued product refinement, such as adding aneuploidy screening by 2022 to make UNITY fully comprehensive. The test is now available nationwide (except where restricted) and in select international markets. Through a partnership with Eluthia, UNITY launched in Germany, Austria, Switzerland, and the Netherlands in 2019, marking its first global expansion and establishing UNITY as an international prenatal brand.
6) Northstar Oncology Testing Platform
With UNITY established, BillionToOne extended its QCT technology into liquid biopsy oncology via the Northstar platform, launched in 2022. Northstar focuses on ctDNA-based profiling and response monitoring through two complementary assays.
7) Northstar Select™
Northstar Select provides comprehensive genomic profiling (CGP) from a blood draw, analyzing somatic mutations across key cancer genes to guide targeted therapy selection. Its key advantage is a very low limit of detection, enabled by QCT, allowing detection of low-frequency variants (sub-1% allele fraction) that conventional NGS may miss. This is especially valuable when tumor DNA shedding is minimal or tissue biopsy is impractical. Select is pan-cancer and functions similarly to leading CGP tests but offers greater sensitivity. After initial research use, Select became clinically available by 2023 and is now used for advanced cancers requiring molecular insights.
8) Northstar Response™
Northstar Response enables real-time treatment monitoring, measuring methylated ctDNA to quantify tumor burden. Instead of tracking specific mutations, it uses tissue-agnostic methylation signals, generating a tumor burden score from a simple blood draw. This allows oncologists to see early therapy response—often faster than imaging. Data presented at AACR showed Response can reliably distinguish very small changes in ctDNA (e.g., 0.5% vs. 0.55%). The assay is designed for metastatic disease, where continuous dynamic tracking matters more than binary MRD. Response launched alongside Select, creating a unified workflow for mutation discovery and ongoing monitoring.
Together, Select + Response deliver a comprehensive approach: Select identifies actionable mutations to choose the right treatment, while Response tracks tumor dynamics to manage therapy. Early collaborations (e.g., UCSD lung cancer studies) support clinical validation, and head-to-head data at ASCO 2024 indicated strong sensitivity performance versus certain competitors. As of 2025, Northstar assays are processed at BillionToOne’s central lab, with growing Medicare MolDX coverage and private payor adoption as evidence accumulates.
Other Services
BillionToOne offers genetic counseling, clinician support, and a results portal for both providers and patients. It also participates in clinical research programs, where UNITY and Northstar are used in clinical trials, creating both scientific evidence and service revenue. The R&D roadmap suggests expansion into additional diagnostic verticals, potentially including early cancer detection and rare disease screening—areas well suited to the core QCT platform.
UNITY introduced a new model for prenatal testing, combining multiple high-value insights in one test, while Northstar aims to bring more sensitive tumor measurement to the oncology workflow. Both reflect BillionToOne’s mission of quantifying biology to create powerful diagnostics, with adoption demonstrated by hundreds of thousands of patients tested and strong revenue growth. Future iterations will likely expand testing scope and indications, strengthening the overall platform.
Conclusion
BillionToOne’s journey from a Stanford lab to a rising diagnostics leader is defined by breaking scientific limits and turning them into clinical reality. In less than a decade, the company reimagined prenatal care with UNITY by enabling single-gene screening and aneuploidy testing from one maternal blood draw—removing the need for routine paternal testing and reducing invasive procedures. The same molecular counting technology now powers the Northstar oncology platform, allowing oncologists to measure tiny changes in tumor DNA that were once considered impossible to detect. Guided by a mission to make diagnostics accurate, fast, and accessible, BillionToOne has empowered high-risk pregnancies with clearer answers and given clinicians new tools to guide treatment in real time.
From a business perspective, BillionToOne stands out for its focused execution and strategic market expansion. It identified a major gap in prenatal testing, proved its value with strong clinical data and cost-effectiveness, and then scaled the same technology into oncology—dramatically increasing its market potential. As of 2025, the company is at a pivotal stage: rising revenues, improving margins, a growing test menu, and a potential IPO ahead. Competition and regulatory hurdles remain, but BillionToOne’s combination of deep innovation and disciplined growth creates a strong foundation for long-term success. Its story reflects how a clear mission, technological conviction, and patient-centric purpose can reshape standards of care—one molecule at a time.
Also Read: acceldata – Founders, Business Model, Funding & Competitors
To read more content like this, subscribe to our newsletter




